Clinical Practice Guidelines Early Breast Cancer ESMO 2023
Cliquer sur l'image pour agrandir
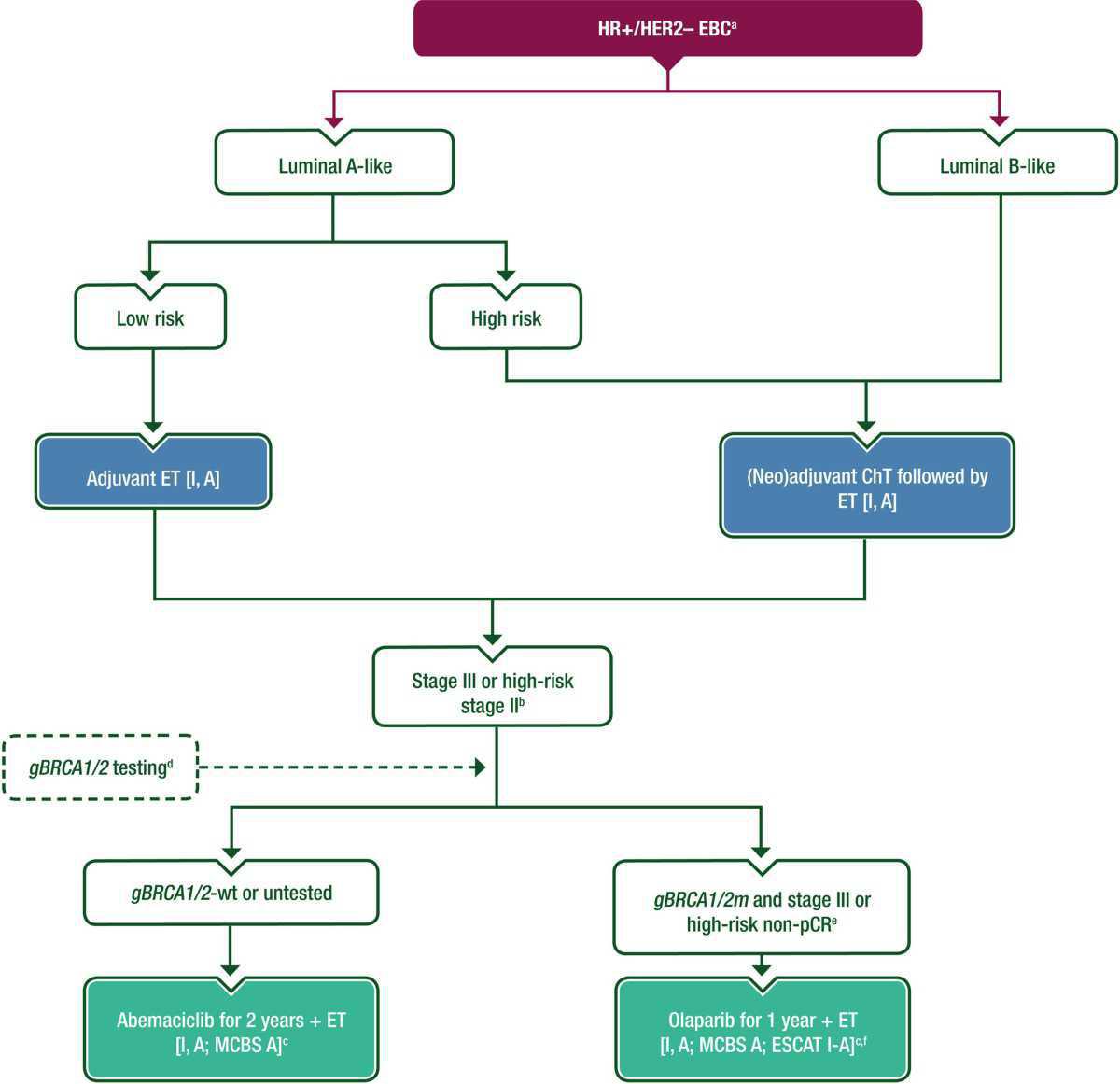
Purple: general categories or stratification; turquoise: combination of treatments or other systemic treatments; white: other aspects of management; blue: systemic anticancer therapy. ChT, chemotherapy; CPS+EG, pretreatment clinical stage and post-treatment pathological stage, estrogen receptor and tumour grade; EBC, early breast cancer; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of molecular Targets; ET, endocrine therapy; FDA, Food and Drug Administration; gBRCA1/2; germline BRCA1/2; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; m, mutation; MCBS, ESMO-Magnitude of Clinical Benefit Scale; N, node; pCR, pathological complete response; wt, wild type.
- aSee Figure 2 for the role of surgery in HR-positive, HER2-negative EBC.
- bStage N1 with primary tumour >5 cm, and/or grade 3 and/or Ki-67 ≥20%.
- cESMO-MCBS v1.1115 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated and validated by the ESMO-MCBS Working Group and reviewed by the authors (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).
- d If gBRCA1/2 testing is appropriate and feasible. ePatients with HR-positive tumours and non-pCR after neoadjuvant ChT require a CPS+EG score ≥3 to receive olaparib.118 fESCAT scores apply to alterations from genomic-driven analyses only. These scores have been defined by the guideline authors and assisted as needed by the ESMO Translational Research and Precision Medicine Working Group.114 See Supplementary Table S7, available at Annals of Oncology online, for more information on ESCAT scores.
Management of HER2-positive EBC (figure 6)