Clinical Practice Guidelines Early Breast Cancer ESMO 2023
Cliquez sur l'image pour agrandir
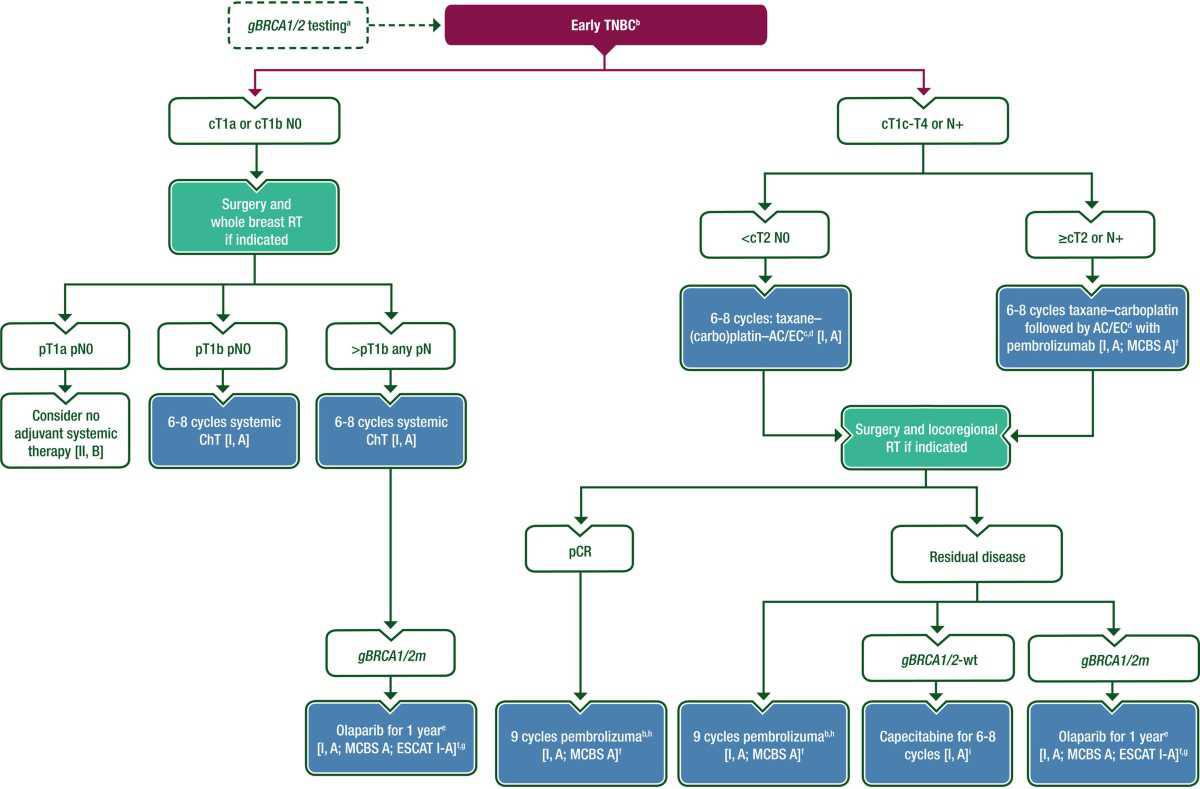
Purple: general categories or stratification; turquoise: combination of treatments or other systemic treatments; white: other aspects of management.; blue: systemic anticancer therapy; dashed line: optional recommendation. AC, doxorubicin–cyclophosphamide; c, clinical; ChT, chemotherapy; CPG, Clinical Practice Guideline; EC, epirubicin–cyclophosphamide; EMA, European Medicines Agency; ER, estrogen receptor; ESCAT, ESMO Scale for Clinical Actionability of molecular Targets; ER; estrogen receptor; ET, endocrine therapy; FDA, Food and Drug Administration; gBRCA1/2, germline BRCA1/2; G-CSF, granulocyte colonystimulating factor; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; ICI, Immune checkpoint inhibitor; m, mutation; MCBS, ESMO-Magnitude of Clinical Benefit Scale; N, node; p, pathological; pCR, pathological complete response; PgR, progesterone receptor; RT, radiotherapy; T, tumour; TNBC, triplenegative breast cancer; wt, wild type.
- aSee the ESMO CPG for risk reduction and screening of cancer in hereditary breastovarian cancer syndromes. 4
- bHER2– tumours with 1%-9% ER and/or PgR expression (ER-low/PgR-low) are a heterogenous group, some of which behave biologically similarly to TNBC; therapeutic strategies should be adjusted to this specific situation since this might lead to a higher response to ChT and to reduced efficacy of ET compared with classical HR+ breast cancer [II, B].
- cThese evidence-based regimens without ICIs are sequential: anthracycline-based therapy followed by a taxane or taxane–carboplatin or vice versa.
- dThe use of dose-dense schedules of ChT, with G-CSF support, should be considered given their documented benefit over non-dose-dense schedules [I, A].
- e Indicated as adjuvant therapy for patients with gBRCA1/2m tumours and non-pCR or ≥pT2 or ≥pN1 if treated with initial surgery.
- fESMO-MCBS v1.1115 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated and validated by the ESMO-MCBS Working Group and reviewed by the authors (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).
- gESCAT scores apply to alterations from genomic-driven analyses only. These scores have been defined by the guideline authors and assisted as needed by the ESMO Translational Research and Precision Medicine Working Group.114 See Supplementary Table S7, available at Annals of Oncology online, for more information on ESCAT scores. hOnly if pembrolizumab was given preoperatively.
- iOnly for ICI-naïve patients.